
The world is at the dawn of an era that might see COVID-19 relegated to the ranks of a mild inconvenience thanks to a rapidly developed vaccine.
This is made possible due to huge advances in medical science made possible through bleeding edge technology. However, before any medical advancement, clinical trials must be conducted to ensure the treatment is safe for humans. For that we need clinical trial management system/software.
Immediate access to accurate, up-to-date information
Healthcare, technical and a variety of other professionals require immediate access to accurate up to date information about their clinical trials. This access can be for something critical, like an updated site roster, or something simple and granular such as startup tracking or site visit schedules. Without immediate access to such information, the entire process risks falling apart.
To remedy this, users should have the capability of immediately uploading information no matter where they are. With a solution like Folderit, you have complete access to the entire platform as long as you are on a smart device with an active internet connection.
This form of controlled transparency and uniform access to data is necessary for the successful implementation of clinical trial management system/software.

Collaboration
A clinical trial management system/software is an ideal platform for a study team to work together as it also enabled cross-team collaboration. This way you can jointly update, work with, and collaborate with sponsors, Contract Research Organizations, and sites.
Collaboration can be enabled through the use of users and user groups that can be granted access to different files and folders throughout the system. You can even set it up such that some users have limited access, while others have complete access to data. This way, all stakeholders are assured they have the latest information.
Oversight
Any stakeholders in this process will need complete access to the following critical components to ensure that everything is in order and progress is being made.
- Study startup
- Screening & enrollment
- Document collection
- Site visits
- Monitoring reports
- Subject visit completion
- Action items and issues
- Management
…and more. If you are collecting data using Microsoft Excel, that can also be integrated through Folderit’s collaboration with Microsoft Office 365 (separate subscription required) to create visualizations and performance scoring for one study or aggregated views of multiple studies.
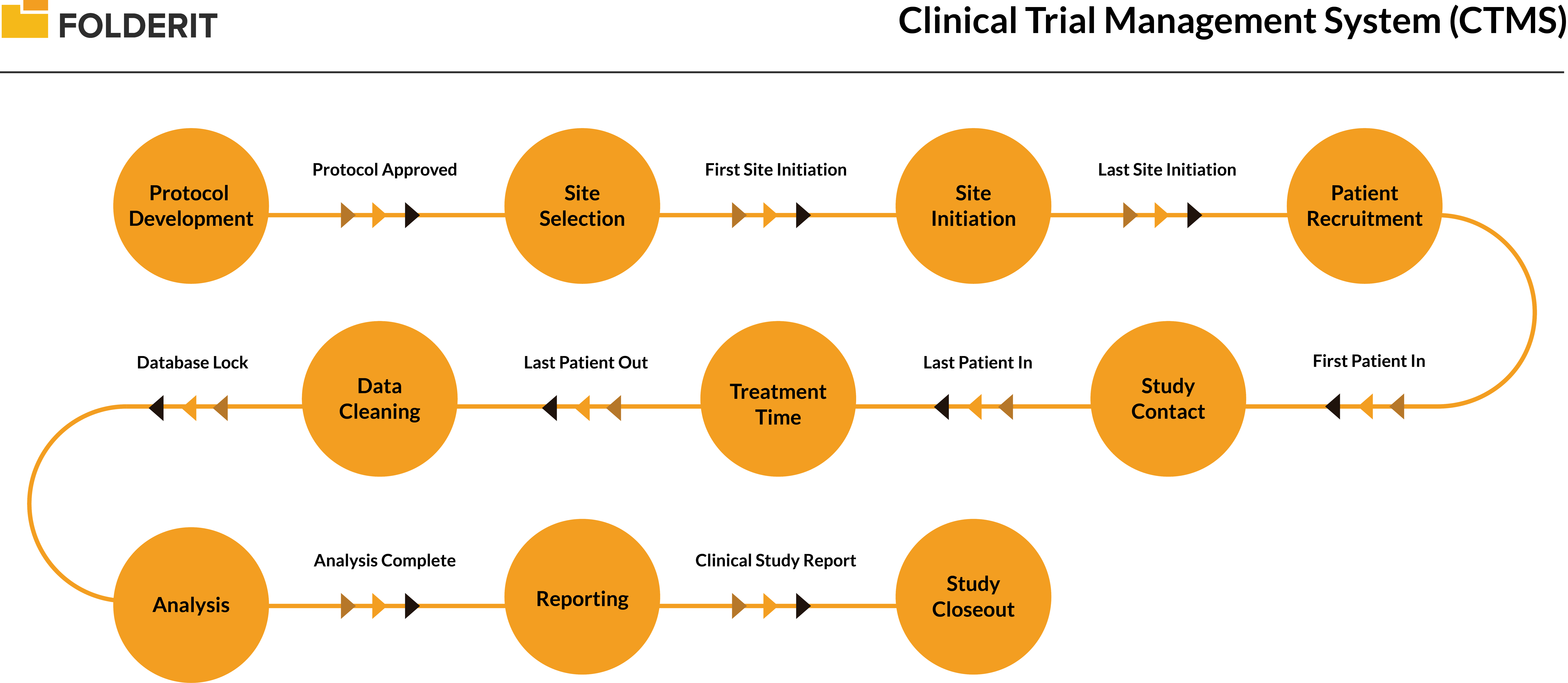
Additional Features important to clinical trial management system/software (CTMS)
A feature rich CTMS needs to offer the following solutions:
Audit Trail and Versioning
A reliable CTMS should be able to manage a complete audit trail for any changes and updates made in the system, including information on who made the change, and when. With versioning, you can even monitor which values have changed over time.
Archival Policy
At times, it is necessary to remove records from your collection; a reliable solution should have the option of how long to keep a file or folder on record with a timer that can span as long as you want, after which the file will automatically cease to exist.
Privacy and Security
One of the most critical bits of information in medical practice is a patient’s personal information. The only way to secure it is to ensure that only authorized users have access to the records and nobody else. You can configure the system such that even certain types of stakeholders don’t have direct access to patient personal records. Additionally, securing the process under a 256-bit bank-level encryption that transfers data over SSL means to protect it from any unauthorized access.
Approvals and Signatures
Approvals are an unavoidable byproduct of clinical trials, the absence of which make for an unreliable system. You need approvals for the entire process from planning and tracking, to any updates in meds, or even for items that might not directly be relevant to regulatory agencies (like day-to-day affairs). Folderit allows for automatic approvals that can be acquired at the same time, or as part of a hierarchy.
Conclusion
The purpose of a clinical trial is to test a medical solution on a number of people to make sure that the medication, or vaccine is safe for large scale consumption. This study and approach are the corner stone of modern medicine and it needs a powerful solution like Folderit to support its critical aspects.



